The EACR’s ‘Highlights in Cancer Research’ is a regular summary of the most interesting and impactful recent papers in cancer research, curated by the Board of the European Association for Cancer Research (EACR).
The list below appears in no particular order, and the summary information has been provided by the authors unless otherwise indicated.
Use the dropdown menu or ‘Previous’ and ‘Next’ buttons to navigate the list.
Hoover, G. et al. Nature. 644: 252–262. (2025).
doi: 0.1038/s41586-025-09176-8.
 Summary of the findings
Summary of the findings
This study reveals that nerves metabolically support cancer progression by directly transferring mitochondria to tumor cells. Using neuron–cancer cocultures and in vivo lineage tracing, we show that neuronal mitochondria integrate into cancer cells, enhancing their respiratory capacity, stemness phenotype, and oxidative metabolism associated with improved redox balance and survival under common metastatic stressors such as oxidative and shear stresses. Chemical denervation with Botox in situ demonstrates the prevalence of these transfers in vivo and confirms that neural input is essential for maintaining cancer cell bioenergetics. Using the MitoTRACER genetic engineering approach, which permanently labels the transfer of mitochondria between cells and enables their fate to be tracked in vivo, we identified a distinct population of “recipient” cancer cells that are significantly enriched at metastatic sites, confirming that mitochondrial transfer provides a selective advantage during dissemination. Together, these findings identify the nerve–cancer interface as a key metabolic conduit that fuels metastasis and introduce MitoTRACER as a powerful tool to trace organelle exchange both in vitro and in complex tissues. By uncovering how Botox-induced denervation disrupts this mitochondrial support, this work establishes a conceptual and therapeutic framework to target cancer metabolism through neural modulation.
.
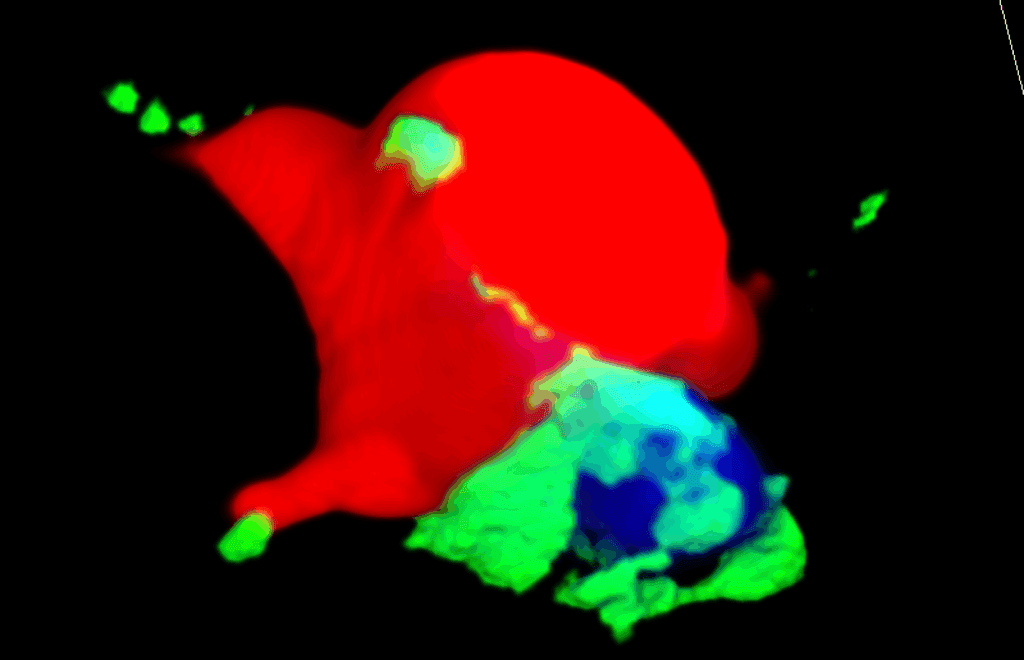
.
Future impact
This study establishes a new understanding of metastatic dissemination by revealing that neuronal mitochondria transfer fuels the metabolic fitness required for cancer spread. Previous approaches could not conditionally and permanently label these transfer events or distinguish recipient from naïve cancer cells. The MitoTRACER system overcomes this limitation, enabling the long-term tracking of recipient cells and the identification of those that seed metastases. By targeting this nerve–cancer metabolic axis through Botox denervation or inhibition of mitochondrial transfer, metastasis could be selectively restrained. This work redefines metastasis as a neuro-metabolic process amenable to therapeutic modulation.









